Members Highlights: authored by Qiang Xu
- Article:Catalytic activities of non-noble metals for hydrogen generation from aqueous ammonia–borane at room temperature
- Source Information
- Original Title:Catalytic activities of non-noble metals for hydrogen generation from aqueous ammonia–borane at room temperature
- Authors:Qiang Xu, Manish Chandra
- Affiliations:National Institute of Advanced Industrial Science and Technology (AIST), 1-8-31 Midorigaoka, Ikeda, Osaka 563-8577, Japan
- Keywords:Cobalt; Nickel; Copper; Hydrogen generation; Catalyst; Ammonia–borane
- Source Link:https://www.sciencedirect.com/science/article/pii/S0378775306019458
- Editor’s Comments
This study delivers a timely advance in hydrogen-storage chemistry by demonstrating that earth-abundant transition-metal catalysts (Co, Ni and Cu) can drive the complete hydrolytic dehydrogenation of ammonia-borane at room temperature. The authors replace costly noble metals with inexpensive alternatives and still achieve the stoichiometric release of three equivalents of H2 per mole of ammonia-borane, a benchmark previously confined to platinum-group catalysts. Their systematic comparison across four metals (Co, Ni, Cu, Fe) and three supports (γ-Al2O3, SiO2, Vulcan® carbon) creates an unusually comprehensive kinetic dataset that future techno-economic models can draw upon.
Methodologically, the work combines impregnation–reduction synthesis with multi-scale characterisation (XRD, TEM/EDX, BET). These diagnostics link catalytic activity to particle size and dispersion, revealing that Co nanoparticles (~2.5 nm) dispersed on high-surface-area carbon outperform identically loaded oxides. Such structure-activity insights are critical for rational catalyst design yet are often missing from the ammonia-borane literature.
Kinetic measurements show zero-order behaviour in ammonia-borane and yield an apparent activation energy of 62 kJ mol-1 for Co/γ-Al2O3. This value, lower than those reported for Co-B or Co-only catalysts in NaBH4 hydrolysis, underscores the unique reactivity of ammonia-borane and suggests a different rate-determining step—most likely B–N bond scission at the metal surface. The authors’ brief mechanistic discussion aligns with emerging dehydrocoupling models and invites in-situ spectroscopic verification.
Beyond fundamental insight, the paper has clear practical relevance. At 25 ℃, only 0.27 kg of ammonia-borane is required to sustain a 1 kW PEM fuel-cell stack for one hour, making these catalysts attractive for portable power packs. However, long-term stability, recyclability and by-product management (NH4+/BO2–) remain open questions. Future work should explore bimetallic synergy, continuous-flow reactors and integration with on-demand purification membranes to meet system-level targets for gravimetric and volumetric energy density.
- Original text summary
This article presents a detailed study on the precise measurement of CO₂ emission factors in China’s cement production, highlighting significant discrepancies in previous global and domestic estimates. Through field sampling of 289 production lines across 18 major cement-producing provinces, combined with a bottom-up factory-level sampling method (BFSM), the research distinguishes contributions from process-related, combustion-related, and electricity-related emission sources. Key findings reveal that traditional IPCC defaults and CSI standards likely overestimate China’s cement-related CO₂ emissions due to technological shifts (e.g., transitioning from wet to dry processes), lower-than-assumed limestone content in clinker, dynamic clinker-to-cement ratios, and widespread use of industrial waste substitutes. Case studies show that NSP kilns emit 1–3% less CO₂ per ton of cement compared to traditional shaft kilns, while higher-grade cement formulations exhibit elevated emission factors due to increased clinker content. The study advocates for refined regional emission factor databases and grid-specific electricity emission factors to enhance the accuracy of China’s carbon accounting, providing critical data for global emission inventories and climate policy development.
The article investigates hydrogen generation from 1 wt % aqueous ammonia-borane at room temperature using supported non-noble metals. Co, Ni and Cu (10 wt % on γ-Al2O3) liberate ~2.86–2.87 equivalents of H2 within 65–70 min, whereas Fe remains inactive. Switching the Co support from γ-Al2O3 (13 nm, 52 m2 g-1) to SiO2 (12 nm) yields similar rates, but dispersing 2.5 nm Co particles on Vulcan® carbon (270 m2 g-1) markedly accelerates hydrolysis, confirming that smaller particle size and higher metal surface area enhance activity. All active catalysts produce essentially pure hydrogen with no detectable NH₃ contamination, and ¹¹B NMR spectra show quantitative conversion of ammonia-borane to NH4+/BO2–. Kinetic traces are linear, indicating zero-order dependence on ammonia-borane concentration; Arrhenius analysis between 20 ℃ and 40 ℃ gives Ea = 62 kJ mol-1 for Co/γ-Al2O3. The authors attribute rate control to surface-mediated B–N bond cleavage followed by concerted hydrolysis. They calculate that ammonia-borane offers 0.195 g H2 g-1—competitive with NaBH4 and LiH—and conclude that low-cost Co/Ni/Cu catalysts show strong promise for portable PEM fuel-cell hydrogen supplies.
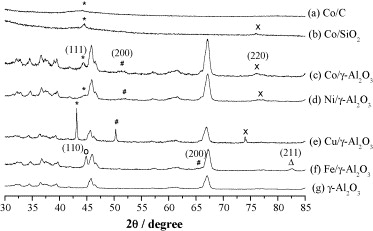
Fig. 1. Powder X-ray diffraction profiles for the supported Co, Ni and Cu catalysts.
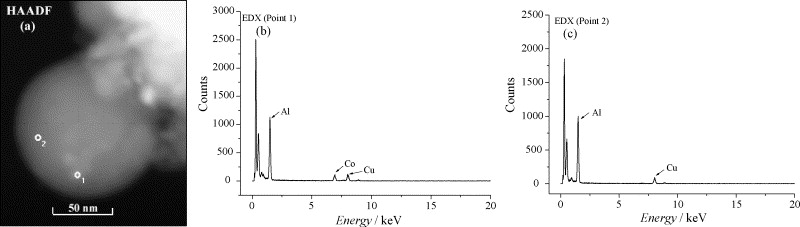
Fig. 2. (a) HAADF image of the Co/γ-Al2O3 (10 wt.%) catalyst and the corresponding EDX spectra at (b) point 1 and (c) point 2 in (a). The Cu signal in (b) and (c) is due to the TEM grid.
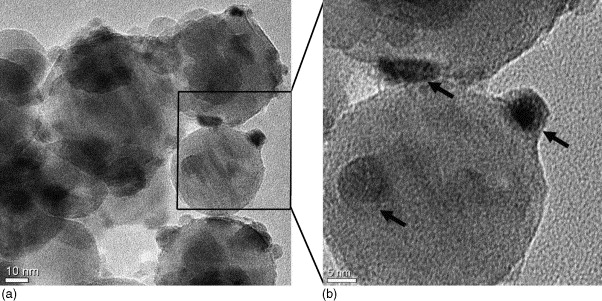
Fig. 3. HRTEM image of Ni/γ-Al2O3 (10 wt.%) catalyst. Arrow indicates nickel particles over large γ-Al2O3 particles.
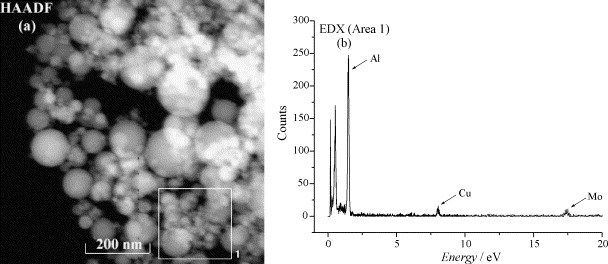
Fig. 4. (a) HAADF image of the Cu/γ-Al2O3 (10 wt.%) catalyst and the corresponding EDX spectrum of area 1 in (a). The Mo signal in (b) is due to the TEM grid.
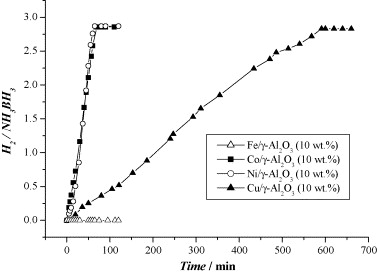
Fig. 5. Hydrogen generation from aqueous NH3BH3 (1 wt.%, 10 ml) in the presence of γ-Al2O3 supported catalysts (metal/NH3BH3 = 0.018).
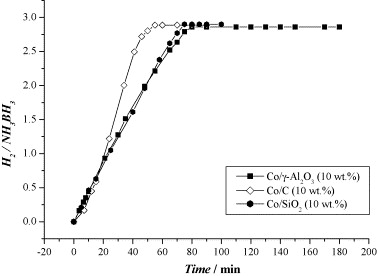
Fig. 6. Hydrogen generation from aqueous NH3BH3 (1 wt.%, 10 ml) in the presence of Co/γ-Al2O3 (10 wt.%), Co/SiO2 (10 wt.%) and Co/C (10 wt.%) (metal/NH3BH3 = 0.018).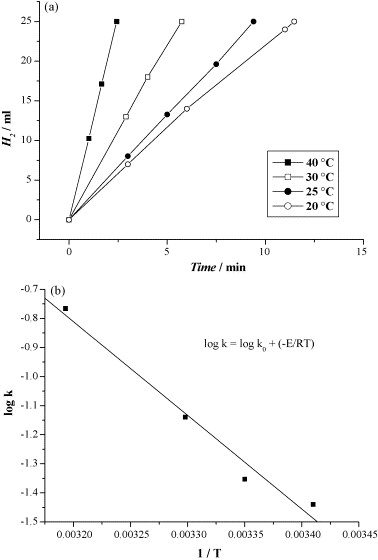
Fig. 7. (a) Hydrogen generation from aqueous NH3BH3 (1 wt.%, 10 ml) in the presence of Co/γ-Al2O3 (10 wt.%) at 20, 25, 30 and 40 ℃ and (b) the log k vs. 1/T plot calculated from (a) (Co/NH3BH3 = 0.018). Calculated activation energy is 62 kJ mol-1.
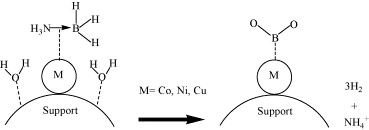
Fig. 8. Schematic representation of hydrolysis of NH3BH3 in the presence of supported metal catalysts.
- Original text information

ABSTRACT
We have studied catalytic performance of supported non-noble metals for hydrogen generation from aqueous NH3BH3 at room temperature. Among the tested non-noble metals, supported Co, Ni and Cu are the most catalytically active, with which hydrogen is released with an almost stoichiometric amount from aqueous NH3BH3, whereas supported Fe is catalytically inactive for this reaction. Support effects on the catalytic activity have been investigated by testing the hydrogen generation reaction in the presence of Co supported on γ-Al2O3, SiO2 and C and it is found that the Co/C catalyst has higher activity. Activation energy for hydrogen generation from aqueous NH3BH3 in the presence of Co/γ-Al2O3 was measured to be 62 kJ mol-1; this may correspond to the step of B—N bond breaking. Particle size, surface morphology and surface area of the supported metal catalysts were examined by X-ray diffraction (XRD), transmission electron microscope (TEM), energy dispersive X-ray (EDX) and BET experiments. It is found that with decreasing the particle size the activity of the supported catalyst is increased. The low-cost and high-performance supported non-noble metal catalysts may have high potential to find its application to the hydrogen generation for portable fuel cells.
- This issue’s editor
Mr Wenhao SUN, Doctoral candidate at Institute of Geographic Science and Natural Resources Research (IGSNRR), the Chinese Academy of Sciences (CAS), focuses on the research of natural resources-energy nexus.
